More applications
FutureChemistry Applications
Droplet Generation in a microreactor
Chemical processes occurring on the fluid/fluid interface can be studied and enhanced by means of efficient droplet generation. An increasing demand is emerging for effortless microdroplet generation. In response to this demand, the viability of our common flow chemistry instruments to be used as microdroplet generator has been analysed by FutureChemistry using our short quench microreactor.
In contrast to homogeneous reaction mixtures, some reactions are carried out on the liquid-liquid interface. Offering a large increase in reaction rate and greatly facilitating work-up as the synthesised product collects in one of the fluid phases. One example is the cleavage of esters at the toluene/water interface, thereby enhancing the reaction rate while continuously extracting the product into the water phase. Thus, a large interfacial area is crucial to reaction speed-up. In batch, droplets can be generated through mechanical stirring. However, continuous flow offers the added advantage of precise control over droplet size and diameter distribution.

contact us for this application note
Remotely flow chemistry
FutureChemistry is also a specialist in producing more customised and dedicated flow systems. In collaboration with the VU University Amsterdam, we developed the FlowStart remote. The FlowStart remote is a flow system that can be used remotely through an online control platform. This custom made system gives high school students access to flow chemistry without safety risks. Students can synthesise, optimise and analyse a pH-dependent dye, methyl orange, from their computer. This web experiment can be accessed from any location in the world with internet access.

Azides
The synthesis of azides is a key reaction in organic chemistry, as it provides a good pathway towards triazoles through the Huisgen azide-alkyne cycloaddition. Traditionally, this reaction is carried out using a variety of azide reagents, which can be explosive and release toxic gas.
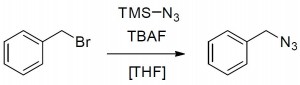
Azide substitution on model substrate using trimethylsilyl azide
In general, azide chemistry is dangerous: depending on substrate, reagent, temperature etc., azides can decompose violently. To be able to handle these potentially explosive compounds in a closed system, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable amine substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Diazo transfer on model substrate using imidazole sulfonyl azide
In general, azide chemistry is dangerous: depending on substrate, reagent, temperature etc., azides can decompose violently. To handle these potentially explosive compounds in a closed system, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable amine substrate:
Please contact us for more information on this application notes
– Flow Process Development & Optimisation
Diazotization: Synthesis of Sudan I
The dying of fabric using azo dyes in a continuous process is often performed by treating fabric (soaked in a phenolate solution) with an aryl diazonium compound. This method circumvents the need for dissolving the dye, but suffers from the instability of the diazonium species. However, in a continuous process it is advantageous to synthesise the diazonium compound in a continuous matter as well, without having to store the diazonium solution under freezing conditions to prevent denitrogenation.
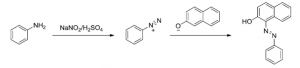
Synthesis of Sudan I
To avoid decomposition of the diazonium compound while keeping a high throughput at the same time, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable
substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download the following application notes
– Flow Process Development & Optimisation
– Out scaling to kg/day production
Wittig Reaction
A convenient and selective way of forming a carbon-carbon double bond is through the Wittig reaction and its modifications. In this example, a two-phase system was used. The increased surface-to-volume ratio in the microreactor led to increased mass transport and faster overall reaction rate.

Customers have access to our portal to download the application notes. Please contact us for more info on this application note
– Flow Process Development & Optimisation.
In situ generation of phosphonium ylides
A convenient and selective way of forming a carbon-carbon double bond is through the Wittig reaction and its modifications. Conventionally, this reaction is a two-step process, where the Wittig salt is treated with a base to obtain the reactive phosphonium ylide species, with subsequent workup and addition of the aldehyde. Using base-stable compounds, the reaction can be performed as a one-pot procedure as well. In batch, this two-phase system suffers from irreproducibility issues due to uncontrollable mixing behaviour. In continuous flow chemistry, this translates to a plug-flow system with the advantage of added control over the two-phase system.

Wittig reaction to form tert-Butyl cinnamate
In a feasibility study, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Scaling up to preparative synthesis.
Log in to the FutureChemistry Portal to download the following application note
– Flow Process Development & Optimisation.
Enzymatic enantioselective C–C-bond formation in microreactors
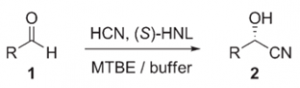
Org. Process Res. Dev., 2009, 13 (5), 1003-1006.
Selective α-ketone bromination
The synthesis of α-ketone bromides is a useful reaction in organic chemistry, as it provides a good pathway towards α-substituted ketones through bromide substitution. Traditionally, this reaction is difficult to control due to its fast reaction rate and exothermic character. As a result, the brominated ketone easily reacts further to the double-brominated product. In batch, side product formation is largely overcome by controlled reagent addition and the use of mild bromination reagents (instead of bromine) such as N-bromosuccinimide, whose synthesis again requires the use of bromine. Direct α-ketone bromination with bromine limits batch scale-up, but has been shown to be possible in continuous flow. The latter has the added advantage of handling all toxic and corrosive reagents inside a closed system.
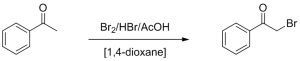
Log in to the FutureChemistry Portal to download the following application notes
To avoid the use of expensive bromination reagents while keeping a high throughput at the same time, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable ketone substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Scaling up to preparative synthesis
– Flow Process Development.
– Automated Optimisation.
– Out scaling to kg/day production.






