Exothermic Reactions
Grignard Reaction
The Grignard reaction is a very useful organic transformation, as it effectively provides a carbanion synthon by treatment of the corresponding halide with magnesium metal. Alternatively, a variety of Grignard reagents is available as commercial solutions. In batch, Grignard reactions have to be conducted at strict anhydrous conditions under an inert atmosphere, as the reagent is very sensitive to atmospheric moisture. Continuous flow solves this issue, as the total system is inherently free of air.

Grignard reaction on model substrate
To avoid the use of inert gasses and dosing equipment while keeping a high throughput at the same time, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application note
– Flow Process Development & Optimisation
Swern-Moffatt oxidation
The Swern-Moffatt oxidation of primary and secondary alcohols to the corresponding aldehydes and ketones is a useful reaction in organic chemistry, as it provides a selective pathway towards aldehydes and ketones. This reaction is difficult to control due to its fast reaction rate and exothermic character, and is therefore traditionally performed at -78°C. At elevated temperatures, side product formation becomes dominant, except when very short reaction times are used. These short reaction times can be perfectly controlled in a continuous flow microreactor, and a minimum of 300 ms was obtained using the short quench microreactor.
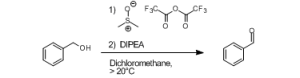
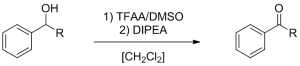
Swern-Moffatt oxidation of model substrates
(R = H, CH3)
To avoid the use of low temperatures and controlled reagent addition, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable alcohol substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
As customer you can log in to the FutureChemistry Portal to download this application notes, Not a customer please contact us for the ful application note
– Flow Process Development & Optimisation
– Out scaling to kg/day production
Nitration of Cumene
The nitration of aromatic compounds is difficult to control due to its exothermic character and the reagents’ high reactivity. Using undiluted nitration mixture the reaction is very prone to thermal runaway and release of large amounts of nitrous fumes. As a result, this reaction is often performed by paralleling batch reactions. This limits batch scale-up, but it is now shown to be possible in continuous flow.
![]()
Log in to the FutureChemistry Portal to download this application notes
– Flow Process Development
– Automated Optimisation
– Out scaling to kg/day production
Halogen-Lithium exchange
The use of n-Butyllithium as lithiation reagent as several drawback since it has to be conducted at strict anhydrous conditions under an inert atmosphere at extreme low temperatures. Furthermore, good yields can be difficult to obtain, as the lithiated compound can even react with the used solvent. Continuous flow solves these issues, by using short reaction times at elevated temperatures.
![]()
Log in to the FutureChemistry Portal to download this application note
– Flow Process Development
Aldol condenstation: Synthesis of dibenzalacetone
The aldol condensation of benzaldehyde and acetone is a textbook example of an exothermic, spontaneous reaction which is often performed during practical courses at universities and high schools. Due to its exothermic character, the reaction vessel is traditionally cooled in an ice bath, with controlled reagent addition to avoid the formation of side products and evaporation of acetone. The product dibenzalacetone is used as a UV blocker and as a ligand in organometallic chemistry.
![]()
Aldol condensation of benzaldehyde and acetone to dibenzalacetone
To avoid the use of cooling and controlled reagent addition, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable aldol condensation:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application note
– Flow Process Development
Paal-Knorr pyrrole synthesis
The Paal-Knorr pyrrole synthesis was first published in 1885 by Carl Paal and Ludwig Knorr. It is a spontaneous, moderately exothermic reaction, which can also be used in the synthesis of furans and thiophenes. Due to its exothermic nature, the reaction is of not much use in the chemical industry, since batch scale-up reaches its limits very quickly.
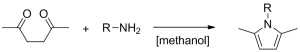
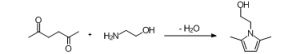
Paal-Knorr pyrrole synthesis
(R = ethyl; 2-hydroxyethyl; 4-hydroxybutyl)
The Paal-Knorr pyrrole synthesis is a fast and exothermic reaction, which limits the feasibility of batch process up-scaling. FutureChemistry has therefore translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable amine/diketone substrate couple:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application notes
– Flow Process Development
– Automated Optimisation
– Out scaling to kg/day production
Deprotection of p-methoxyphenyl (PMP) protected amines
In the synthesis of enantiopure amines, p-methoxyphenyl (PMP) is often the most suitable protecting group, since the protection-deprotection sequence does not change the amine stereochemistry. Most literature procedures describe the deprotection with ceric ammonium nitrate, which involves column chromatography and produces highly toxic waste. Recently, a mild and efficient deprotection using periodic acid was reported requiring only acid/base extraction, leading to an overall reduction of costs and a more environmentally benign process.
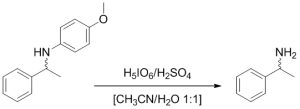
Deprotection of model substrate
Oxidative cleavage of the PMP group is a fast and exothermic reaction, which limits the feasibility of batch process up-scaling. FutureChemistry has therefore translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable PMP protected amine substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application notes
– Flow Process Development
– Automated Optimisation
– Out scaling to kg/day production
Nitration of Anisole
The nitration of aromatic compounds is a key reaction in organic chemistry. Conventionally, this reaction is difficult to control due to its exothermic character and the reagents’ high reactivity. As a result, the reaction is often performed by paralleling batch reactions. Direct nitration with nitric acid limits batch scale-up, but it is now shown to be possible in continuous flow. The latter has the added advantage of handling all toxic and corrosive reagents inside a closed system.
![]()
Nitration of model substrate
To avoid the use of expensive nitration reagents while keeping a high throughput at the same time, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application notes
– Flow Process Development & Optimisation
– Out scaling to kg/day production
Methylation with diazomethane
Reactions employing diazomethane as a methylating agent are very useful in organic synthesis, due to their high selectivity and yields. However, preparing diazomethane from one of the available precursor compounds requires much care, since it is a highly reactive species which can spontaneously explode on contact with sharp edges. Also, the reagent is very toxic, requiring good ventilation and safety measures to handle any unwanted liberation of diazomethane.
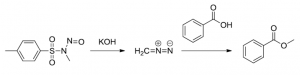
Methylation of benzoic acid with diazomethane
In general, diazomethane chemistry is dangerous. To be able to handle this explosive and toxic compound in a closed system, FutureChemistry has translated this reaction from a batch process to a continuous flow process. FutureChemistry’s typical three-tier approach led to a protocol which can be adapted to any viable substrate:
1) Translation of batch process to continuous flow process:
a) Stock solutions approach, yielding a homogeneous reaction mixture.
b) Quenching solution to follow the reaction in time.
c) Flow markers approach to accurately assess reaction parameters.
2) Automated reaction optimisation.
2) Automated reaction optimisation.
3) Out scaling to preparative synthesis
Log in to the FutureChemistry Portal to download this application note
– Flow Process Development & Optimisation
Epoxidation Prilezhaev reaction of alkenes and formation of trans-diols
The synthesis of epoxides is a useful reaction in organic chemistry, as it provides a good pathway towards trans-diols through alkaline hydrolysis. Traditionally, this reaction is difficult to control due to its fast reaction rate and exothermic character.
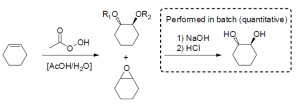
Log in to the FutureChemistry Portal to download this application notes
– Flow Process Development
– Automated Optimisation
– Out scaling to kg/day production






